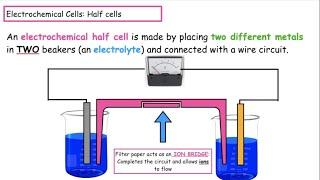
Live Class - O'level/IGCSE/GCSE Chemistry - Metals Part 1
Описание
For Free Live Demo Classes, Register at
https://megalecture.com/live-subject-classes/
Timestamps:
0:00 - Metals' reaction with acid
6:00 - Metals' reaction with water
19:31 - Rusting of iron
22:00 - How to prevent rusting
In this video we discuss the reactivity series for metals and cover their reactions with acid and water. We also discuss the process of iron rusting and ways to prevent it.
The reactivity series ranks metals by how readily they react. More reactive metals displace less reactive metals from their compounds and react with water. Displacement reaction is the displacement of ions of metal from compounds of metals lower in reactivity series by metals higher in reactivity series.
When a metal reacts with acid, it produces a salt and hydrogen gas. The more reactive the metal, the more hydrogen gas is produced per second. Therefore the more reactive the metal, the more bubbles being produced. When a metal reacts with water, a metal hydroxide and hydrogen are formed.
Rusting is the corrosion of iron and steel where a brown solid product is formed. We will discuss 5 methods of preventing a metal from rusting in this video.
For more Video Lectures for O Levels, A Levels, IB Diploma, AP Courses & Edexcel:
https://www.megalecture.com
https://www.youtube.com/megalecture
For Zoom/Whiteboard Subject Experts and Tutors and Free Online Trial Classes, Contact:
megalecture@gmail.com